OTT1368
Technology
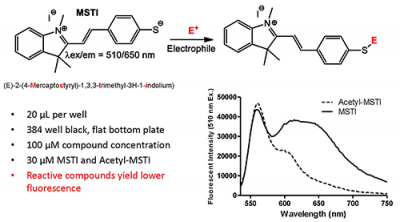
The inventors have developed a novel fluorescence-based high throughput assay, for the detection of thiol-reactive thus electrophilic drug candidates that are likely to irreversibly interact with biological targets. These promiscuous inhibitors can be identified rapidly, in parallel, for small molecule screening libraries using 384 or 1536 well plate formats. Testing small molecules for their ability to modulate cysteine residues of proteins in the early stages of drug discovery is expected to increase efficiency and success of every HTS campaign.
Thiol-containing molecules have an essential role in many biochemical and physiological reactions due to the ease with which they are oxidized and form new bonds. Glutathione is the most abundant non-protein thiol as it is found in the millimolar range in most cells. Therefore, it is important to determine the reactivity of the small molecules toward thiols to circumvent unwanted side-effects of potential drug candidates. Currently, there are no established pre-clinical high throughput assays for the identification of electrophilic compounds that potentially react with glutathione and other thiol-containing biomolecules. With the use of intrinsically fluorescent nucleophilic probe, these promiscuous inhibitors can be sensitively and selectively identified among screening compounds.
Features/Benefits
- More accurate – Less interference from molecules due to use of far red spectrum
- Faster – Can be used for high throughput screening (1536-well plate format)
- More versatile – Identifies both electrophilic and redox reactive compounds
- Stable product – Use of acetylated precursor allows for storage of the assay probe and its reliable generation in situ
Intellectual Property
A U.S. Provisional Patent Application has been filed for this technology. This technology is available for use through a research use license.
Markets
Drug discovery is integral to a pharmaceutical company’s business strategy. There are many new technologies being used in the industry such as bioanalytical equipment, genomics, pharmacogenomics, combinatorial chemistry, biochip, proteomics, bioinformatics and high throughput screening (HTS). Global Industry Analysts Inc. published in 2008 that the market is dominated by the US and Europe.
Global Information, Inc. reported the global market for drug discovery technologies and products at $41.4B in 2012 and predicted to $79B 2017. The HTS market is expected to grow from $11.5 B in 2012 to $20B in 2017. Drug development lead time has not followed the rapid development of these tools showing the gap between the initial introduction of new platforms and their further application and commercial success. Over the next 10 years the pharmaceutical industry will seek new ways to improve and accelerate the drug development process.
Inventor (s)
Alexander Arnold, Assistant Professor in the Department of Chemistry and Biochemistry at the University of Wisconsin-Milwaukee
Megan McCallum & Premchendar Nandhikonda
Publications
McCallum, M.M., Nandhikonda P., Temmer, C.E., Simeonove, A., Jadhav, A., Yasgar, A., Maloney, M., and A. Arnold. 2013. High-Throughput Identification of Promiscuous Inhibitors from Screening Libraries with the Use of a Thiol-Containing Fluorescent Probe. Journal of Biomolecular Screening 18(6): 705-713.