OTT1287
Technology
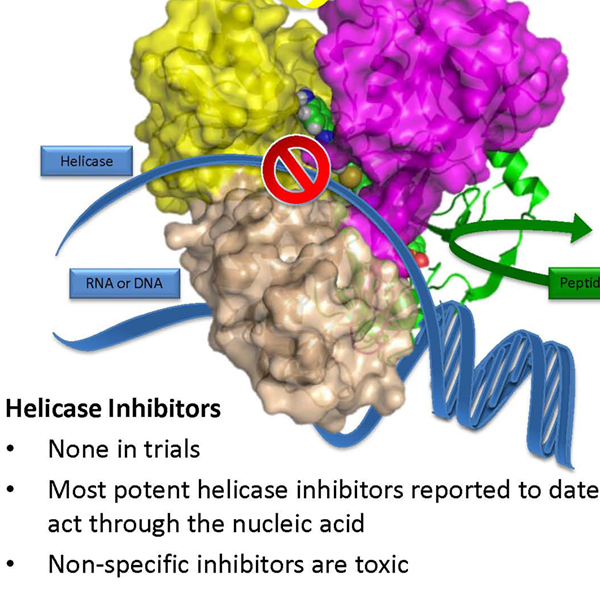
The invention consists of new direct acting antivirals (DAAs) that act against the Hepatitis C virus (HCV) replicon and inhibit the NS3 helicase activity. These DAAs are additionally unique compared to other HCV helicase inhibitors because they are also capable of inhibiting HCV NS3 protease activity.
HCV needs a functional helicase to replicate in cells. While helicases have been widely studied as possible drug targets, progress has been slow compared to other viral enzymes because the most potent compounds are non-specific, and inhibit normal healthy cellular processes. HCV is typically treated with various combinations of the nucleoside analog ribavirin combined with one of several recombinant human alpha interferons. Though such treatments are effective, therapy is poorly tolerated, expensive, and not equally effective against all HCV genotypes. DAAs typically are small molecules that inhibit viral enzymes. DAAs are considered better HCV treatments because, unlike interferon and ribavirin, DAAs directly attack proteins that HCV synthesizes in human cells.
Two DAAs, telaprevir and boceprevir, were recently approved for use in HCV patients, both are protease inhibitors but neither alone eradicates HCV infection because HCV rapidly evolves to become resistant to them. The protease inhibitors need to be administered with interferon and ribavirin, and consequently many patients still poorly tolerate the new therapies. Therefore, new DAAs for HCV as described here are needed that might be used with telaprevir, boceprevir or similar drugs to replace interferon and ribavirin in HCV therapy.
Features/Benefits
- Multiple effects – Inhibits both the helicase and protease activities of HCV NS3
- Broad Acting – Inhibit all HCV genotypes with similar potency
- Fewer potential side effects – No detectable toxicity in cell culture
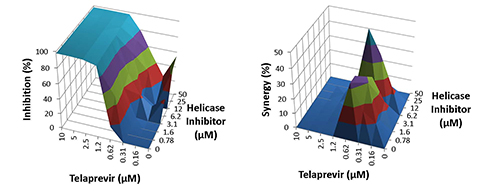
- Compatible with other DAAs– Enhance the effects of telaprevir
- Combination Potential – Shows synergy in combination with other drugs on market
Intellectual Property
United States patent application US20140227225 A1. This technology is available for developmental research support/licensing under either exclusive or nonexclusive terms.
Markets
Hepatitis C in combination with hepatitis B, accounts for about 75% of all liver disease around the world. 170-200 million people are infected with HCV worldwide with 3-5 million in the USA. Chronic HCV infection develops in 70%–85% of HCV-infected persons. 60%–70% of chronically infected persons have evidence of active liver disease.
The global Hepatitis C virus market is attractive due to high levels of unmet need. The unmet need in the market is approximately 70%, which equals about $3 billion. Some of this market availability is due to the lack of effective options for treatment as well as the lack of treatments with good efficacy and moderate safety profiles. Serious side effects still exist with a majority of the single and combination treatments used today. The global Hepatitis C market was worth approximately $4.4 billion in in 2009. It is expected to reach $9.8 billion by 2016.
*www.epidemic.org; www.imaginggenetics.net “Hepatitis C-Drug pipeline analysis and market forecasts to 2016; http://www.businesswire.com/news/home/20110817005291/en/Research-Markets-Global-Hepatitis-Virus-HCVMarket; http://www.cdc.gov/hepatitis/HCV/
Inventor (s)
David Frick, Jeffrey Aube, Frank Schoenen, Brian Blagg, Kevin Frankowski, Kelin Li
Dr. David Frick is an Associate Professor in the Department of Chemistry and Biochemistry at the University of Wisconsin-Milwaukee. He obtained his Ph.D. from the Department of Biology at the Johns Hopkins University. His laboratory studies the enzymes that viruses use to multiply in human cells in order to develop better antiviral drugs, specifically focusing on better treatments for HCV.
Publications
Sweeney NL, Hanson AM, Mukherjee S, Ndjomou J, Geiss BJ, Steel JJ, Frankowski KJ, Li K, Schoenen FJ, Frick DN. ABenzothiazole and Pyrrolone Flavivirus Inhibitors Targeting the Viral Helicase. CS Infect Dis. 2015 Mar 13;1(3):140-148.
Ndjomou J, Corby MJ, Sweeney NL, Hanson AM, Aydin C, Ali A, Schiffer CA, Li K, Frankowski KJ, Schoenen FJ, Frick DN. Simultaneously Targeting the NS3 Protease and Helicase Activities for More Effective Hepatitis C Virus Therapy. ACS Chem Biol. 2015 Aug 21;10(8):1887-96.
Li K, Frankowski KJ, Hanson AM, Ndjomou J, Shanahan MA, Mukherjee S, Kolli R, Shadrick WR, Sweeney NL, Belon CA, Neuenswander B, Ferguson J, Aubé J, Schoenen FJ, Blagg BSJ, Frick DN. Hepatitis C Virus NS3 Helicase Inhibitor Discovery. Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010-.2011 Dec 16.
Sweeney NL, Shadrick WR, Mukherjee S, Li K, Frankowski KJ, Schoenen FJ, Frick DN. Primuline derivatives that mimic RNA to stimulate hepatitis C virus NS3 helicase-catalyzed ATP hydrolysis. J Biol Chem. 2013 Jul 5;288(27):19949-57.
Ndjomou J, Kolli R, Mukherjee S, Shadrick WR, Hanson AM, Sweeney NL, Bartczak D, Li K, Frankowski KJ, Schoenen FJ, Frick DN. Fluorescent primuline derivatives inhibit hepatitis C virus NS3-catalyzed RNA unwinding, peptide hydrolysis and viral replicase formation. Antiviral Res. 2012 Nov;96(2):245-55.
Li K, Frankowski KJ, Belon CA, Neuenswander B, Ndjomou J, Hanson AM, Shanahan MA, Schoenen FJ, Blagg BS, Aubé J, Frick DN. Optimization of potent hepatitis C virus NS3 helicase inhibitors isolated from the yellow dyes thioflavine S and primuline. J Med Chem. 2012 Apr 12;55(7):3319-30