Applications
Proteomics, protein electrophoresis
Target Problems
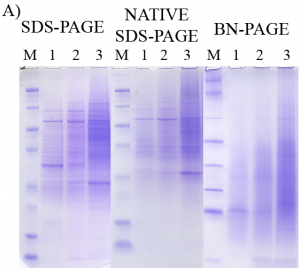
In traditional SDS-PAGE, proteins are well separated during electrophoresis but are denatured such that the structure and function are no longer adequately maintained for carrying out further functional assays. Those wishing to retain protein functional behavior, must use non-denaturing (native) techniques, which suffer from inferior resolution.
Key Features
- Better Resolution: Electrophoretic resolution is superior to traditional native PAGE methods.
- Activity Maintained: Researchers can conduct further experiments with the electrophoresis products unlike with standard SDS-PAGE.
- Quick to Market: Compatible with many popular precast gels. Only small adjustments to sample buffer and run buffers and SDS-PAGE kits currently in production will be required for the new product.
- Inexpensive Development: Minimal costs would be necessary to develop the “kit” for distribution.
- Numerous Applications: Useful for proteomics works, drug discovery, diagnostics, personalized medicine, protein-based therapeutics, and toxicology.
Technology
The patented invention featured here – Native SDS-PAGE – allows researchers to retain the functional behavior of proteins without sacrificing resolution in gel electrophoresis. This is a robust method and buffer system that has the added benefit of working with many precast electrophoresis gels and chambers that are already available on the market.
Native SDS-PAGE allows proteins to be both well separated and maintain their native 3-dimensional conformations and functional activity. After electrophoresis, native proteins are ready for isolation from the gel for further analysis, direct in-gel assay of enzymatic activity, direct in-gel survey of binding activity of proteins with small molecules (e.g. drugs, toxic agents) and macromolecules (e.g. protein binding partners including antibodies, cognate DNA binding sites), and isolation of protein for subsequent mass spectral identification. This easy to implement Native SDS-PAGE method has retained the enzymatic activity of a number of enzymes tested and has also maintained protein complexes in a bound state.
Intellectual Property
About the Inventor (s)
David Petering, William Wobig, Andrew Nowakowski
Dr. David Petering Distinguished Professor at the University of Wisconsin-Milwaukee, Department of Chemistry and Biochemistry
Please contact our office to share your business’ needs and learn more.